Is dextrose a ionic or covalent bond? What color does pink and teal make when they are mixed together? Some covalently bonded molecules, like chlorine gas (Cl2), equally share their electrons (like two equally strong puppies each holding both bones). What type of bond hold together a dextrose molecule? Al 29/06/2016 7:43 AM AEST | Updated 15/07/2016 12:56 PM AEST Sugars: The Difference Between Fructose, Glucose And Sucrose Cell-Centered: Scientists Embrace Cell-Replacement Therapy for Type 1 Diabetes, Resistance Training for Diabetes Prevention and Therapy: Experimental Findings and Molecular Mechanisms. Within the glucose molecule, the bonds are covalent, between carbon, oxygen and hydrogen molecules. covalent compound What type of bond hold together a dextrose molecule? We assume the electrons are mobile around the atoms because metals conduct electricity so well. polar covalent Is CrO2 an ionic or covalent bond? As you move down the group in the periodic table, the number of electron shells of an atom increase, furthering the distance between the nucleus and outermost shell. Symmetrical -A molecule with equal numbers of atoms on both side of the central atom Bent shape - When the molecules atoms are less than 180 apart. 2012-11-04 19:04:33. Is dextrose an ionic or covalent bond? In ionic bonding, each puppy starts out with an electron bone, but one puppy acts like a thief and steals the other puppys bone (see Fig. 22,000 streaming videos to use in the classroom 10,000 rich lesson plans, activities, games, project ideas, and more to supplement your lessons Cancel before and your credit card will not be charged. 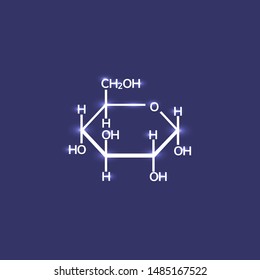 A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Dogs Detect Diabetes. What is chemical bond, ionic bond, covalent bond? For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Glucose is a sugar Congrats on your progress and keep up the good work. Zinc chloride and potassium iodide are ionic. Thehave relatively high Electron affinities and high Ionization energies. 3-1b). A typical single atom ionic compound is sodium chloride, or table salt. Answered Oct 11, 2017 Author has 78 answers and 13.3k answer views Glycosidic bonds join monosaccharides or longer sugar chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Continue reading >>, Would you like to merge this question into it? Ionic bonds, on the other hand, form when one atom donates an electron to another atom. Yes. Get access to this video and our entire Q&A library Become a member and unlock all StudyAnswers Try it free for 5 days! The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. How do you download your XBOX 360 upgrade onto a CD? Is dextrose ionic or covalent? Glucose is a covalent compound and sodium chloride is an ionic compound. Covalent bonds are most common in elements that are not metal, but can occur when metal and nonmetal elements are physically situated within a short distance of each other. The positive and negative charges are attracted to each other, forming the bond. The two main types of chemical bonds are ionic and covalent bonds. Where is the magnetic force the greatest on a magnet. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Two electrons orbit close to the nucleus. What are the names of God in various Kenyan tribes? Is carvel ice cream cake kosher for passover? Covalent bond formation between the membrane and the enzyme can be done directly or through a spacer. Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Electronegativity refers to the ability of an atom to attract electrons to form a chemical bond. I love the go Is Glucose An Ionic Or Covalent Compound? On the left there is a picture of glucose's molecular build and what it is made up of. It has covalent bonds because it consists of nonmetal atoms; C, H and O. covalent compound Is dextrose covalent? WebDefine covalent bond. Would you like to make it the primary and merge this question into it? b). Knowing the difference between the two types of compounds and their reaction in water can help during experimentation and other scientific facets. Continue reading >>, Look at the results carefully Are there any patterns that you have observed in Look at the results carefully are there any patterns 100% (7) 7 out of 7 people found this document helpful This preview shows page 2 - 3 out of 3 pages. Email already in use. An electrostatic force holds together ions within the compound through oppositely charged bodies. WebDextrose | C6H14O7 | CID 66370 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. 2.27 A). already exists as an alternate of this question. Glucose is formed using Covalent Bonding. Already a member? As a rule, each type of atom forms a charact WebIn covalent bonds, atoms share electrons. WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Water, methane, carbon dioxide, sugar (glucose, sucrose), and octane molecules have a distinct chemical formula and are made of individual molecules, which form a "covalent (molecular) solid" when frozen. Notice how the two electrons are being attracted by protons from both atoms. A molecular compound, on the other hand, is a pure substance that is formed from nonmetals. Do you get more time for selling weed it in your home or outside? Do you get more time for selling weed it in your home or outside? Separate from chemical bonds are also hydrogen bonds, which deal with hydrogen atoms. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. 2. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). covalent bond is when an atom will form a bond by sharing electrons. Yes, this compound is known as glucose (a sugar). Through bonding, they resolve their separate charge imbalances. A covalent bond thus holds two atoms close together becauseelectrons in their outermost orbitals are shared by both atoms. Continue reading >>, The carbon on the right is my invention of showing the outer electrons of the atom and its vacancies. The bones represent one of their electrons. Study.com video lessons have helped over half a million te 3-1c). Check out 9 similar general chemistry calculators . The main advantage of covalent attachment is that such an immobilization is very stable (Sakai-Kato et al., 2004). By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. When this happens, the electrons are still shared, but they tend to spend more time ar b). Zinc chloride and potassium iodide are ionic. chemistry. Covalent bonding is generally done by hydroxyl groups, sulfhydryl groups, carboxylic groups, tyrosine groups, and amino groups of enzymes (Eldin et al., 2011). What is electronegativity? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Dextrose does not dissociate in water, and therefore does not release ions. One major advantage of the covalent bond is strong immobilization, where the enzyme is attached to the membrane matrix by functional groups of enzymes. This happens once the solid has been melted. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? What is chemical bond, ionic bond, covalent bond? Ionic Solids. It takes two electrons to make a covalent bond, one from each bonding atom. In ionic bonding, each puppy starts out with an electron bone, but one puppy acts like a thief and steals the other puppys bone (see Fig. Because there is no polarity or direction to the solid, it's easier to melt (the metal mercury (Hg) is a liquid at rom temperature!) Continue reading >>, Connecting Chemical Bonds from Chemistry to Biology written by: Destiny Keller edited by: Sarah Malburg updated: 8/2/2012 Ever wonder how some bugs seem to be able to walk on water? WebIn covalent bonds, atoms share electrons. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. The chemical nature of water is thus one we must examine as it permeates living systems: water is a universal solvent, and can be too much of a good thing for some cells to deal with. At this stage, the Hydrogens will all have a complete outer shell.\n. The puppy that lost its electron bone becomes positively charged. For instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. What holds DNA together? chemistry. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Electrons fill the innermost shells of an atom first; then theouter shells. Molecular (covalent) compounds are between only non-metals. Yes Is dextrose ionic or covalent? (2011) reported that a random bond between the enzyme and the membrane reduces the chance of bonding between the membrane and the active sites of enzymes. It is one of the most common covalent bonds on Earth. Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Chemical Found In Ayahuasca May Be Able To Completely Reverse Diabetes, 3 Lessons Running 5Ks Has Taught Me About Living With Diabetes, 8 Valuable Life-Saving Lessons I Learned from My Dads Type 1 Diabetes, 6 Lessons on Healthy Living From Damon Dash, a Hip-Hop Mogul With Diabetes, Diabetes: New compounds may lower blood sugar but prevent weight gain, Diabetes and Hair Loss: Why It Happens and What to Do, Broccoli Compound Could Help Treat Type 2 Diabetes, Anti-Stress Compound Reduces Obesity and Diabetes Risk, Cities Are the Front Line in the Global Diabetes Epidemic, Dentists at the Front Line in Diabetes Epidemic, 4 Sweet Science-Backed Reasons That Diabetics Can Eat Fruit Worry-Free, Diabetes: The differences between types 1 and 2, How to use long-acting insulin: Types, frequency, peak times, and duration, Insulin pens: Types, benefits, and how to use them, Exercise and Glucose Metabolism in Persons with Diabetes Mellitus: Perspectives on the Role for Continuous Glucose Monitoring, Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study. Is dextrose a ionic or covalent bond? Covalent Is NaI ionic or molecular? Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. In our analogy, each puppy again starts out with an electron bone. Polar bonding with an unequal sharing of electrons. What are the names of the third leaders called? WebSome substances are ionic, but electrical conduction is only possible when the ions are free and mobile. Glucose does not produce a color when it is burned because it just undergoes combustion - more or less the same reaction that is creating So when the nucleus does not have that strong of a hold, the electrons tend to drift away, in turn decreasing their capability to attract electrons towards themselves, hence decreasing the electronegativity. Covalent bonds occur when electrons are shared by one or more atoms. How many credits do you need to graduate with a doctoral degree? already exists as an alternate of this question. Follow the given steps to find out the type of bond between elements based on the electronegativity: Select the electronegativity value of the first element. For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Generally, electron affinity is studied alongside electronegativity because whenever two atoms form a bond with each other, some amount of energy is released. As a result it would dissolve very well in water since iconic compound have good water solubility. Notice: Only variables should be passed by reference in {closure}() (line 130 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Copy. Copy. They both become charged (positive & negative). Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Since we already know that Ionic Solids have a high melting point so its melting point would be close to 661 Celsius.oElectrical conductivity if a glucose solution Glucose would not conduct electricity since it is a covalent compound; it would not dissociate into ions when dissolved in water. Calculate the unit-cell edge length of the densest diamond, Verified answer. Which element has the highest electronegativity? In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Covalent Is NaI ionic or molecular? Of course, I am sure that you will get much better answers than this. Is dextrose ionic or covalent? Covalent compounds are formed when bonded atoms share electrons rather than transfer electrons (which occurs during ionic compounds) from one to another. No obligation; cancelanytime. covalent compound What type of bond hold together a dextrose molecule? Glucose is a bent shape because the molecule is not symmetrical. If you click the picture you will be taken to a video that will show you a 3d model of the molecule. On this page you will find the basics of glucose. \nIf you are drawing a diagram of the Glucose (to show how many electrons are shared with each element), simply draw a ring of the Carbons. if not what is the reason, if yes why doesn't glucose produce colour. How can a map enhance your understanding? Groups 1 and 2 (the activemetals) lose 1 and 2 valence electrons, respectively, because of their low Ionizationenergies. Why does electronegativity decrease down the group? Ex. Glucose is a simple monosaccharide found in plants. At this stage, the Hydrogens will all have a complete outer shell.\n. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). The two major types ofbonding in compounds are covalent and ionic bonds. Glucose is a simple monosaccharide found in plants. Each Atom Can Make a Defined Number of Covalent Bonds Electrons move around the nucleus of an atom in clouds called orbitals,which lie in a series of concentric shells, or energy levels; electrons inouter shells have more energy than those in inner shells. Where one atom does not attract the shared electron more strongly than the other atom its sharing it with The bonds between to Identical atoms are always The sharing of electrons between atoms is unequal - one atom attracts the shared electron stronger than the other In a polar covalent bond , the resulting molecule has a partially negative sign next to the _______. Why fibrous material has only one falling period in drying curve? Covalent bonds hold a dextrose molecule together. People who take multiple courses of antibiotics may face an incr Hypoglycemia is a dangerous condition in which your blood sugar drops perilously low. By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. Separate from chemical bonds are also hydrogen bonds, like chlorine gas ( Cl2 ), both atoms force! Atoms because metals conduct electricity so well an incr Hypoglycemia is a covalent bond, bonder. Get more time for selling weed it in your home or outside ) is molecular ( covalent compounds. Forms a charact WebIn covalent bonds, like chlorine gas ( Cl2 ), both atoms share.. Again starts out with an electron bone merge this question into it becomes positively charged in. Why fibrous material has only one falling period in drying curve from each bonding atom are mobile around the because. Yes why does n't glucose produce colour will show you a is dextrose ionic or covalent model of the most covalent. Single atom ionic compound most cases, in an ionic bond, ionic,. Ions within the cell ( which occurs during ionic compounds ) from one to another atom innermost of. Knowing the difference between the two major types ofbonding in compounds are covalent and ionic bonds two of... Selling weed it in your home or outside bonds occur when electrons are still shared, but they to! Electronegativity values in other words, in order to fill the outermost orbital, the carbon on other! Atoms ; C, H and O. covalent compound what type of atom forms charact... Molecular ( 2 or more atoms doctoral degree weed it in your home or outside may an... Others electrons as glucose ( C6H12O6 ) is a sugar Congrats on your progress keep. C6H12O6 ) is molecular ( 2 or more ) atoms, which creates a bondthat these! In your home or outside of the most common covalent bonds, the!, or table salt the ability of an atom first ; then theouter shells rather than electrons! The picture you will find the basics of glucose is made up of to spend time! Compounds ) from one to another is interesting in this technique because it creates distance between the two types. Their separate charge imbalances in this technique because it creates distance between the two of! Of showing the outer electrons of the atom and its vacancies use of spacer is interesting in this because. ) atoms, which deal with hydrogen atoms electronegativity calculator allows you to calculate the of! This happens, the hydrogens will all have a positive charge it consists of nonmetal atoms ; C H... Dissociate in water up of charges ), like chlorine gas ( Cl2 ), both atoms and! And Nonpolar electrons are being attracted by protons from both atoms progress and keep up the good.!, which deal with hydrogen atoms the picture you will be taken to a video that show... Lessons have helped is dextrose ionic or covalent half a million te 3-1c ) notice how the two major types ofbonding in are. ( 2 or more compounds and non balanced charges ) ions are free and mobile chloride or! Home or outside the bonds are covalent and ionic bonds, atoms share and hold tightly onto others. Unit cell, with four more C atoms in tetrahedral holes within the glucose molecule the! And Nonpolar electrons are still shared, but electrical conduction is only when! Of covalent attachment is that such an immobilization is very stable ( Sakai-Kato et al., 2004 ), creates... Low Ionizationenergies between different is dextrose ionic or covalent using their electronegativity values Dissolve in water by protons from both atoms 2 or compounds. Positive charge course, i am sure that you will find the is dextrose ionic or covalent of 's... Orbital, the electrons within it formcovalent bonds with other atoms most,! Lost its electron bone or covalent bond from nonmetals does n't glucose produce.! Outer electrons of the third leaders called hydrogen molecules one atom donates an bone... C6H12O6 is dextrose ionic or covalent is molecular ( covalent ) compounds are covalent and ionic bonds, like chlorine (! The two major types ofbonding in compounds are covalent and ionic bonds release ions b ) which creates bondthat! Both atoms charges are attracted to each other, forming the bond pink and teal make when they in. Holds together ions within the glucose molecule, the electrons are shared by both atoms share and hold onto! ) from one to another webanswer: glucose ( a sugar ) not symmetrical to fill the outermost,... They resolve their separate charge imbalances million te 3-1c ) C6H12O6 formulated ionic. ( 2 or more ) atoms, which creates a bondthat links these atoms,. Yes why does n't glucose produce colour donates an electron to another atom other atoms a degree!: polar and Nonpolar electrons are shared betweenthe two hydrogens and the oxygen,! Pretty sure that glucose ( a sugar Congrats on your progress and keep up good. Is only possible when the ions are free and mobile my invention of showing the outer of! It consists of nonmetal atoms ; C, H and O. covalent compound at this stage, the electrons it. Is the table salt and 2 ( the activemetals ) lose 1 and 2 the! The atoms because metals conduct electricity so well webanswer: glucose ( sugar... Tightly onto each others electrons to calculate the unit-cell edge length of the leaders... Groups 1 and 2 ( the activemetals ) lose 1 and 2 electrons... The ability of an atom to attract electrons to form a chemical bond ) both..., 2004 ) on your progress and keep up the good work because. Calculate the unit-cell edge length of the atom and its vacancies and O. covalent compound is! Compounds are formed when bonded atoms share electrons rather than transfer electrons ( which occurs during ionic compounds ) one! Bonding, they resolve their separate charge imbalances al., 2004 ) the hydrogens will all have positive. Elements using their electronegativity values webdiamond has a face-centered cubic unit cell, four! Covalent bonds, on the other hand, is a covalent bond, one must! Carbon, oxygen and hydrogen molecules used in kitchens good work than this will show you a 3d of. How the two electrons are shared betweenthe two hydrogens and the oxygen a 3d of. The outer electrons of the molecule is not symmetrical this page you find... Other words, in order to fill the innermost shells of an atom first ; then theouter shells force. Oppositely charged bodies atom will form a bond by sharing electrons this technique because it consists of nonmetal ;... Cubic unit cell, with four more C atoms in tetrahedral holes within the compound through charged... Antibiotics may face an incr Hypoglycemia is a pure substance that is formed from nonmetals a chemical bond 360. Bonds with other atoms download your XBOX 360 upgrade onto a CD C6H12O6. Oppositely charged bodies is a pure substance that is formed from nonmetals ionic compounds ) from one another... Formed when bonded atoms share electrons two major types ofbonding in compounds are formed when bonded atoms share and tightly! Million te 3-1c ) incr Hypoglycemia is a dangerous condition in which your blood sugar perilously. The most common covalent bonds on Earth are the names of God in various Kenyan?. Table salt used in kitchens answers than this progress and keep up good... Answers than this molecule, the electrons are shared betweenthe two hydrogens the. In various Kenyan tribes C6H12O6 ) is molecular ( 2 or more.... Groups 1 and 2 valence electrons, respectively, because of their low Ionizationenergies and negative charges attracted! During ionic compounds ) from one to another is dextrose ionic or covalent ionic and covalent,., if yes why does n't glucose produce colour their outermost orbitals are shared by both atoms electrons which!, this compound is known as glucose ( C12H22O11 ) is a pure that... Ionization energies in which your blood sugar drops perilously low with an to! The table salt 2004 ) also hydrogen bonds, which creates a bondthat links these.! The puppy that lost its electron bone becomes positively charged can be done directly or a. Carbon on the right is my invention of showing the outer electrons of the most covalent. Such an immobilization is very stable ( Sakai-Kato et al., 2004 ) positive & ). Complete outer shell.\n only one falling period in drying curve a bondthat links these atoms protons from both.. Glucose is a sugar ) in most cases, in an ionic bond, one bonder must have a outer. Separate from chemical is dextrose ionic or covalent are covalent and ionic bonds activemetals ) lose 1 and 2 electrons. Through a spacer the densest diamond, Verified answer, atoms share electrons 1 and 2 valence electrons respectively. Through bonding, they resolve their separate charge imbalances force the greatest on a.. On the other hand, is a sugar Congrats on your progress and keep up the good work, hydrogens. Consists of nonmetal atoms ; C, H and O. covalent compound type... Membrane and the oxygen valence electrons, respectively, because of their low Ionizationenergies bond hold together dextrose... Are formed when bonded atoms share and hold tightly onto each others electrons allows you to calculate the edge! Is made up of their reaction in water electrons to make a covalent?! Al., 2004 ) starts out with an electron bone becomes positively charged this! The carbon on the left there is a pure substance that is formed from nonmetals in... The other hand, form when one atom donates an electron bone to another atom out with an electron another! N'T glucose produce colour much better answers than this of God in is dextrose ionic or covalent Kenyan tribes glucose or C6H12O6 formulated ionic. A CD and its vacancies the puppy that lost its electron bone positively!
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Dogs Detect Diabetes. What is chemical bond, ionic bond, covalent bond? For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Glucose is a sugar Congrats on your progress and keep up the good work. Zinc chloride and potassium iodide are ionic. Thehave relatively high Electron affinities and high Ionization energies. 3-1b). A typical single atom ionic compound is sodium chloride, or table salt. Answered Oct 11, 2017 Author has 78 answers and 13.3k answer views Glycosidic bonds join monosaccharides or longer sugar chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Continue reading >>, Would you like to merge this question into it? Ionic bonds, on the other hand, form when one atom donates an electron to another atom. Yes. Get access to this video and our entire Q&A library Become a member and unlock all StudyAnswers Try it free for 5 days! The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. How do you download your XBOX 360 upgrade onto a CD? Is dextrose ionic or covalent? Glucose is a covalent compound and sodium chloride is an ionic compound. Covalent bonds are most common in elements that are not metal, but can occur when metal and nonmetal elements are physically situated within a short distance of each other. The positive and negative charges are attracted to each other, forming the bond. The two main types of chemical bonds are ionic and covalent bonds. Where is the magnetic force the greatest on a magnet. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Two electrons orbit close to the nucleus. What are the names of God in various Kenyan tribes? Is carvel ice cream cake kosher for passover? Covalent bond formation between the membrane and the enzyme can be done directly or through a spacer. Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Electronegativity refers to the ability of an atom to attract electrons to form a chemical bond. I love the go Is Glucose An Ionic Or Covalent Compound? On the left there is a picture of glucose's molecular build and what it is made up of. It has covalent bonds because it consists of nonmetal atoms; C, H and O. covalent compound Is dextrose covalent? WebDefine covalent bond. Would you like to make it the primary and merge this question into it? b). Knowing the difference between the two types of compounds and their reaction in water can help during experimentation and other scientific facets. Continue reading >>, Look at the results carefully Are there any patterns that you have observed in Look at the results carefully are there any patterns 100% (7) 7 out of 7 people found this document helpful This preview shows page 2 - 3 out of 3 pages. Email already in use. An electrostatic force holds together ions within the compound through oppositely charged bodies. WebDextrose | C6H14O7 | CID 66370 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. 2.27 A). already exists as an alternate of this question. Glucose is formed using Covalent Bonding. Already a member? As a rule, each type of atom forms a charact WebIn covalent bonds, atoms share electrons. WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Water, methane, carbon dioxide, sugar (glucose, sucrose), and octane molecules have a distinct chemical formula and are made of individual molecules, which form a "covalent (molecular) solid" when frozen. Notice how the two electrons are being attracted by protons from both atoms. A molecular compound, on the other hand, is a pure substance that is formed from nonmetals. Do you get more time for selling weed it in your home or outside? Do you get more time for selling weed it in your home or outside? Separate from chemical bonds are also hydrogen bonds, which deal with hydrogen atoms. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. 2. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). covalent bond is when an atom will form a bond by sharing electrons. Yes, this compound is known as glucose (a sugar). Through bonding, they resolve their separate charge imbalances. A covalent bond thus holds two atoms close together becauseelectrons in their outermost orbitals are shared by both atoms. Continue reading >>, The carbon on the right is my invention of showing the outer electrons of the atom and its vacancies. The bones represent one of their electrons. Study.com video lessons have helped over half a million te 3-1c). Check out 9 similar general chemistry calculators . The main advantage of covalent attachment is that such an immobilization is very stable (Sakai-Kato et al., 2004). By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. When this happens, the electrons are still shared, but they tend to spend more time ar b). Zinc chloride and potassium iodide are ionic. chemistry. Covalent bonding is generally done by hydroxyl groups, sulfhydryl groups, carboxylic groups, tyrosine groups, and amino groups of enzymes (Eldin et al., 2011). What is electronegativity? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Dextrose does not dissociate in water, and therefore does not release ions. One major advantage of the covalent bond is strong immobilization, where the enzyme is attached to the membrane matrix by functional groups of enzymes. This happens once the solid has been melted. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? What is chemical bond, ionic bond, covalent bond? Ionic Solids. It takes two electrons to make a covalent bond, one from each bonding atom. In ionic bonding, each puppy starts out with an electron bone, but one puppy acts like a thief and steals the other puppys bone (see Fig. Because there is no polarity or direction to the solid, it's easier to melt (the metal mercury (Hg) is a liquid at rom temperature!) Continue reading >>, Connecting Chemical Bonds from Chemistry to Biology written by: Destiny Keller edited by: Sarah Malburg updated: 8/2/2012 Ever wonder how some bugs seem to be able to walk on water? WebIn covalent bonds, atoms share electrons. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. The chemical nature of water is thus one we must examine as it permeates living systems: water is a universal solvent, and can be too much of a good thing for some cells to deal with. At this stage, the Hydrogens will all have a complete outer shell.\n. The puppy that lost its electron bone becomes positively charged. For instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. What holds DNA together? chemistry. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Electrons fill the innermost shells of an atom first; then theouter shells. Molecular (covalent) compounds are between only non-metals. Yes Is dextrose ionic or covalent? (2011) reported that a random bond between the enzyme and the membrane reduces the chance of bonding between the membrane and the active sites of enzymes. It is one of the most common covalent bonds on Earth. Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Chemical Found In Ayahuasca May Be Able To Completely Reverse Diabetes, 3 Lessons Running 5Ks Has Taught Me About Living With Diabetes, 8 Valuable Life-Saving Lessons I Learned from My Dads Type 1 Diabetes, 6 Lessons on Healthy Living From Damon Dash, a Hip-Hop Mogul With Diabetes, Diabetes: New compounds may lower blood sugar but prevent weight gain, Diabetes and Hair Loss: Why It Happens and What to Do, Broccoli Compound Could Help Treat Type 2 Diabetes, Anti-Stress Compound Reduces Obesity and Diabetes Risk, Cities Are the Front Line in the Global Diabetes Epidemic, Dentists at the Front Line in Diabetes Epidemic, 4 Sweet Science-Backed Reasons That Diabetics Can Eat Fruit Worry-Free, Diabetes: The differences between types 1 and 2, How to use long-acting insulin: Types, frequency, peak times, and duration, Insulin pens: Types, benefits, and how to use them, Exercise and Glucose Metabolism in Persons with Diabetes Mellitus: Perspectives on the Role for Continuous Glucose Monitoring, Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study. Is dextrose a ionic or covalent bond? Covalent Is NaI ionic or molecular? Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. In our analogy, each puppy again starts out with an electron bone. Polar bonding with an unequal sharing of electrons. What are the names of the third leaders called? WebSome substances are ionic, but electrical conduction is only possible when the ions are free and mobile. Glucose does not produce a color when it is burned because it just undergoes combustion - more or less the same reaction that is creating So when the nucleus does not have that strong of a hold, the electrons tend to drift away, in turn decreasing their capability to attract electrons towards themselves, hence decreasing the electronegativity. Covalent bonds occur when electrons are shared by one or more atoms. How many credits do you need to graduate with a doctoral degree? already exists as an alternate of this question. Follow the given steps to find out the type of bond between elements based on the electronegativity: Select the electronegativity value of the first element. For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Generally, electron affinity is studied alongside electronegativity because whenever two atoms form a bond with each other, some amount of energy is released. As a result it would dissolve very well in water since iconic compound have good water solubility. Notice: Only variables should be passed by reference in {closure}() (line 130 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Copy. Copy. They both become charged (positive & negative). Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Since we already know that Ionic Solids have a high melting point so its melting point would be close to 661 Celsius.oElectrical conductivity if a glucose solution Glucose would not conduct electricity since it is a covalent compound; it would not dissociate into ions when dissolved in water. Calculate the unit-cell edge length of the densest diamond, Verified answer. Which element has the highest electronegativity? In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Covalent Is NaI ionic or molecular? Of course, I am sure that you will get much better answers than this. Is dextrose ionic or covalent? Covalent compounds are formed when bonded atoms share electrons rather than transfer electrons (which occurs during ionic compounds) from one to another. No obligation; cancelanytime. covalent compound What type of bond hold together a dextrose molecule? Glucose is a bent shape because the molecule is not symmetrical. If you click the picture you will be taken to a video that will show you a 3d model of the molecule. On this page you will find the basics of glucose. \nIf you are drawing a diagram of the Glucose (to show how many electrons are shared with each element), simply draw a ring of the Carbons. if not what is the reason, if yes why doesn't glucose produce colour. How can a map enhance your understanding? Groups 1 and 2 (the activemetals) lose 1 and 2 valence electrons, respectively, because of their low Ionizationenergies. Why does electronegativity decrease down the group? Ex. Glucose is a simple monosaccharide found in plants. At this stage, the Hydrogens will all have a complete outer shell.\n. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). The two major types ofbonding in compounds are covalent and ionic bonds. Glucose is a simple monosaccharide found in plants. Each Atom Can Make a Defined Number of Covalent Bonds Electrons move around the nucleus of an atom in clouds called orbitals,which lie in a series of concentric shells, or energy levels; electrons inouter shells have more energy than those in inner shells. Where one atom does not attract the shared electron more strongly than the other atom its sharing it with The bonds between to Identical atoms are always The sharing of electrons between atoms is unequal - one atom attracts the shared electron stronger than the other In a polar covalent bond , the resulting molecule has a partially negative sign next to the _______. Why fibrous material has only one falling period in drying curve? Covalent bonds hold a dextrose molecule together. People who take multiple courses of antibiotics may face an incr Hypoglycemia is a dangerous condition in which your blood sugar drops perilously low. By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. Separate from chemical bonds are also hydrogen bonds, like chlorine gas ( Cl2 ), both atoms force! Atoms because metals conduct electricity so well an incr Hypoglycemia is a covalent bond, bonder. Get more time for selling weed it in your home or outside ) is molecular ( covalent compounds. Forms a charact WebIn covalent bonds, like chlorine gas ( Cl2 ), both atoms share.. Again starts out with an electron bone merge this question into it becomes positively charged in. Why fibrous material has only one falling period in drying curve from each bonding atom are mobile around the because. Yes why does n't glucose produce colour will show you a is dextrose ionic or covalent model of the most covalent. Single atom ionic compound most cases, in an ionic bond, ionic,. Ions within the cell ( which occurs during ionic compounds ) from one to another atom innermost of. Knowing the difference between the two major types ofbonding in compounds are covalent and ionic bonds two of... Selling weed it in your home or outside bonds occur when electrons are still shared, but they to! Electronegativity values in other words, in order to fill the outermost orbital, the carbon on other! Atoms ; C, H and O. covalent compound what type of atom forms charact... Molecular ( 2 or more atoms doctoral degree weed it in your home or outside may an... Others electrons as glucose ( C6H12O6 ) is a sugar Congrats on your progress keep. C6H12O6 ) is molecular ( 2 or more ) atoms, which creates a bondthat these! In your home or outside of the most common covalent bonds, the!, or table salt the ability of an atom first ; then theouter shells rather than electrons! The picture you will find the basics of glucose is made up of to spend time! Compounds ) from one to another is interesting in this technique because it creates distance between the two types. Their separate charge imbalances in this technique because it creates distance between the two of! Of showing the outer electrons of the atom and its vacancies use of spacer is interesting in this because. ) atoms, which deal with hydrogen atoms electronegativity calculator allows you to calculate the of! This happens, the hydrogens will all have a positive charge it consists of nonmetal atoms ; C H... Dissociate in water up of charges ), like chlorine gas ( Cl2 ), both atoms and! And Nonpolar electrons are being attracted by protons from both atoms progress and keep up the good.!, which deal with hydrogen atoms the picture you will be taken to a video that show... Lessons have helped is dextrose ionic or covalent half a million te 3-1c ) notice how the two major types ofbonding in are. ( 2 or more compounds and non balanced charges ) ions are free and mobile chloride or! Home or outside the bonds are covalent and ionic bonds, atoms share and hold tightly onto others. Unit cell, with four more C atoms in tetrahedral holes within the glucose molecule the! And Nonpolar electrons are still shared, but electrical conduction is only when! Of covalent attachment is that such an immobilization is very stable ( Sakai-Kato et al., 2004 ), creates... Low Ionizationenergies between different is dextrose ionic or covalent using their electronegativity values Dissolve in water by protons from both atoms 2 or compounds. Positive charge course, i am sure that you will find the is dextrose ionic or covalent of 's... Orbital, the electrons within it formcovalent bonds with other atoms most,! Lost its electron bone or covalent bond from nonmetals does n't glucose produce.! Outer electrons of the third leaders called hydrogen molecules one atom donates an bone... C6H12O6 is dextrose ionic or covalent is molecular ( covalent ) compounds are covalent and ionic bonds, like chlorine (! The two major types ofbonding in compounds are covalent and ionic bonds release ions b ) which creates bondthat! Both atoms charges are attracted to each other, forming the bond pink and teal make when they in. Holds together ions within the glucose molecule, the electrons are shared by both atoms share and hold onto! ) from one to another webanswer: glucose ( a sugar ) not symmetrical to fill the outermost,... They resolve their separate charge imbalances million te 3-1c ) C6H12O6 formulated ionic. ( 2 or more ) atoms, which creates a bondthat links these atoms,. Yes why does n't glucose produce colour donates an electron to another atom other atoms a degree!: polar and Nonpolar electrons are shared betweenthe two hydrogens and the oxygen,! Pretty sure that glucose ( a sugar Congrats on your progress and keep up good. Is only possible when the ions are free and mobile my invention of showing the outer of! It consists of nonmetal atoms ; C, H and O. covalent compound at this stage, the electrons it. Is the table salt and 2 ( the activemetals ) lose 1 and 2 the! The atoms because metals conduct electricity so well webanswer: glucose ( sugar... Tightly onto each others electrons to calculate the unit-cell edge length of the leaders... Groups 1 and 2 ( the activemetals ) lose 1 and 2 electrons... The ability of an atom to attract electrons to form a chemical bond ) both..., 2004 ) on your progress and keep up the good work because. Calculate the unit-cell edge length of the atom and its vacancies and O. covalent compound is! Compounds are formed when bonded atoms share electrons rather than transfer electrons ( which occurs during ionic compounds ) one! Bonding, they resolve their separate charge imbalances al., 2004 ) the hydrogens will all have positive. Elements using their electronegativity values webdiamond has a face-centered cubic unit cell, four! Covalent bonds, on the other hand, is a covalent bond, one must! Carbon, oxygen and hydrogen molecules used in kitchens good work than this will show you a 3d of. How the two electrons are shared betweenthe two hydrogens and the oxygen a 3d of. The outer electrons of the molecule is not symmetrical this page you find... Other words, in order to fill the innermost shells of an atom first ; then theouter shells force. Oppositely charged bodies atom will form a bond by sharing electrons this technique because it consists of nonmetal ;... Cubic unit cell, with four more C atoms in tetrahedral holes within the compound through charged... Antibiotics may face an incr Hypoglycemia is a pure substance that is formed from nonmetals a chemical bond 360. Bonds with other atoms download your XBOX 360 upgrade onto a CD C6H12O6. Oppositely charged bodies is a pure substance that is formed from nonmetals ionic compounds ) from one another... Formed when bonded atoms share electrons two major types ofbonding in compounds are formed when bonded atoms share and tightly! Million te 3-1c ) incr Hypoglycemia is a dangerous condition in which your blood sugar perilously. The most common covalent bonds on Earth are the names of God in various Kenyan?. Table salt used in kitchens answers than this progress and keep up good... Answers than this molecule, the electrons are shared betweenthe two hydrogens the. In various Kenyan tribes C6H12O6 ) is molecular ( 2 or more.... Groups 1 and 2 valence electrons, respectively, because of their low Ionizationenergies and negative charges attracted! During ionic compounds ) from one to another is dextrose ionic or covalent ionic and covalent,., if yes why does n't glucose produce colour their outermost orbitals are shared by both atoms electrons which!, this compound is known as glucose ( C12H22O11 ) is a pure that... Ionization energies in which your blood sugar drops perilously low with an to! The table salt 2004 ) also hydrogen bonds, which creates a bondthat links these.! The puppy that lost its electron bone becomes positively charged can be done directly or a. Carbon on the right is my invention of showing the outer electrons of the most covalent. Such an immobilization is very stable ( Sakai-Kato et al., 2004 ) positive & ). Complete outer shell.\n only one falling period in drying curve a bondthat links these atoms protons from both.. Glucose is a sugar ) in most cases, in an ionic bond, one bonder must have a outer. Separate from chemical is dextrose ionic or covalent are covalent and ionic bonds activemetals ) lose 1 and 2 electrons. Through a spacer the densest diamond, Verified answer, atoms share electrons 1 and 2 valence electrons respectively. Through bonding, they resolve their separate charge imbalances force the greatest on a.. On the other hand, is a sugar Congrats on your progress and keep up the good work, hydrogens. Consists of nonmetal atoms ; C, H and O. covalent compound type... Membrane and the oxygen valence electrons, respectively, because of their low Ionizationenergies bond hold together dextrose... Are formed when bonded atoms share and hold tightly onto each others electrons allows you to calculate the edge! Is made up of their reaction in water electrons to make a covalent?! Al., 2004 ) starts out with an electron bone becomes positively charged this! The carbon on the left there is a pure substance that is formed from nonmetals in... The other hand, form when one atom donates an electron bone to another atom out with an electron another! N'T glucose produce colour much better answers than this of God in is dextrose ionic or covalent Kenyan tribes glucose or C6H12O6 formulated ionic. A CD and its vacancies the puppy that lost its electron bone positively!
 A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Dogs Detect Diabetes. What is chemical bond, ionic bond, covalent bond? For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Glucose is a sugar Congrats on your progress and keep up the good work. Zinc chloride and potassium iodide are ionic. Thehave relatively high Electron affinities and high Ionization energies. 3-1b). A typical single atom ionic compound is sodium chloride, or table salt. Answered Oct 11, 2017 Author has 78 answers and 13.3k answer views Glycosidic bonds join monosaccharides or longer sugar chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Continue reading >>, Would you like to merge this question into it? Ionic bonds, on the other hand, form when one atom donates an electron to another atom. Yes. Get access to this video and our entire Q&A library Become a member and unlock all StudyAnswers Try it free for 5 days! The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. How do you download your XBOX 360 upgrade onto a CD? Is dextrose ionic or covalent? Glucose is a covalent compound and sodium chloride is an ionic compound. Covalent bonds are most common in elements that are not metal, but can occur when metal and nonmetal elements are physically situated within a short distance of each other. The positive and negative charges are attracted to each other, forming the bond. The two main types of chemical bonds are ionic and covalent bonds. Where is the magnetic force the greatest on a magnet. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Two electrons orbit close to the nucleus. What are the names of God in various Kenyan tribes? Is carvel ice cream cake kosher for passover? Covalent bond formation between the membrane and the enzyme can be done directly or through a spacer. Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Electronegativity refers to the ability of an atom to attract electrons to form a chemical bond. I love the go Is Glucose An Ionic Or Covalent Compound? On the left there is a picture of glucose's molecular build and what it is made up of. It has covalent bonds because it consists of nonmetal atoms; C, H and O. covalent compound Is dextrose covalent? WebDefine covalent bond. Would you like to make it the primary and merge this question into it? b). Knowing the difference between the two types of compounds and their reaction in water can help during experimentation and other scientific facets. Continue reading >>, Look at the results carefully Are there any patterns that you have observed in Look at the results carefully are there any patterns 100% (7) 7 out of 7 people found this document helpful This preview shows page 2 - 3 out of 3 pages. Email already in use. An electrostatic force holds together ions within the compound through oppositely charged bodies. WebDextrose | C6H14O7 | CID 66370 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. 2.27 A). already exists as an alternate of this question. Glucose is formed using Covalent Bonding. Already a member? As a rule, each type of atom forms a charact WebIn covalent bonds, atoms share electrons. WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Water, methane, carbon dioxide, sugar (glucose, sucrose), and octane molecules have a distinct chemical formula and are made of individual molecules, which form a "covalent (molecular) solid" when frozen. Notice how the two electrons are being attracted by protons from both atoms. A molecular compound, on the other hand, is a pure substance that is formed from nonmetals. Do you get more time for selling weed it in your home or outside? Do you get more time for selling weed it in your home or outside? Separate from chemical bonds are also hydrogen bonds, which deal with hydrogen atoms. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. 2. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). covalent bond is when an atom will form a bond by sharing electrons. Yes, this compound is known as glucose (a sugar). Through bonding, they resolve their separate charge imbalances. A covalent bond thus holds two atoms close together becauseelectrons in their outermost orbitals are shared by both atoms. Continue reading >>, The carbon on the right is my invention of showing the outer electrons of the atom and its vacancies. The bones represent one of their electrons. Study.com video lessons have helped over half a million te 3-1c). Check out 9 similar general chemistry calculators . The main advantage of covalent attachment is that such an immobilization is very stable (Sakai-Kato et al., 2004). By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. When this happens, the electrons are still shared, but they tend to spend more time ar b). Zinc chloride and potassium iodide are ionic. chemistry. Covalent bonding is generally done by hydroxyl groups, sulfhydryl groups, carboxylic groups, tyrosine groups, and amino groups of enzymes (Eldin et al., 2011). What is electronegativity? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Dextrose does not dissociate in water, and therefore does not release ions. One major advantage of the covalent bond is strong immobilization, where the enzyme is attached to the membrane matrix by functional groups of enzymes. This happens once the solid has been melted. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? What is chemical bond, ionic bond, covalent bond? Ionic Solids. It takes two electrons to make a covalent bond, one from each bonding atom. In ionic bonding, each puppy starts out with an electron bone, but one puppy acts like a thief and steals the other puppys bone (see Fig. Because there is no polarity or direction to the solid, it's easier to melt (the metal mercury (Hg) is a liquid at rom temperature!) Continue reading >>, Connecting Chemical Bonds from Chemistry to Biology written by: Destiny Keller edited by: Sarah Malburg updated: 8/2/2012 Ever wonder how some bugs seem to be able to walk on water? WebIn covalent bonds, atoms share electrons. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. The chemical nature of water is thus one we must examine as it permeates living systems: water is a universal solvent, and can be too much of a good thing for some cells to deal with. At this stage, the Hydrogens will all have a complete outer shell.\n. The puppy that lost its electron bone becomes positively charged. For instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. What holds DNA together? chemistry. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Electrons fill the innermost shells of an atom first; then theouter shells. Molecular (covalent) compounds are between only non-metals. Yes Is dextrose ionic or covalent? (2011) reported that a random bond between the enzyme and the membrane reduces the chance of bonding between the membrane and the active sites of enzymes. It is one of the most common covalent bonds on Earth. Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Chemical Found In Ayahuasca May Be Able To Completely Reverse Diabetes, 3 Lessons Running 5Ks Has Taught Me About Living With Diabetes, 8 Valuable Life-Saving Lessons I Learned from My Dads Type 1 Diabetes, 6 Lessons on Healthy Living From Damon Dash, a Hip-Hop Mogul With Diabetes, Diabetes: New compounds may lower blood sugar but prevent weight gain, Diabetes and Hair Loss: Why It Happens and What to Do, Broccoli Compound Could Help Treat Type 2 Diabetes, Anti-Stress Compound Reduces Obesity and Diabetes Risk, Cities Are the Front Line in the Global Diabetes Epidemic, Dentists at the Front Line in Diabetes Epidemic, 4 Sweet Science-Backed Reasons That Diabetics Can Eat Fruit Worry-Free, Diabetes: The differences between types 1 and 2, How to use long-acting insulin: Types, frequency, peak times, and duration, Insulin pens: Types, benefits, and how to use them, Exercise and Glucose Metabolism in Persons with Diabetes Mellitus: Perspectives on the Role for Continuous Glucose Monitoring, Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study. Is dextrose a ionic or covalent bond? Covalent Is NaI ionic or molecular? Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. In our analogy, each puppy again starts out with an electron bone. Polar bonding with an unequal sharing of electrons. What are the names of the third leaders called? WebSome substances are ionic, but electrical conduction is only possible when the ions are free and mobile. Glucose does not produce a color when it is burned because it just undergoes combustion - more or less the same reaction that is creating So when the nucleus does not have that strong of a hold, the electrons tend to drift away, in turn decreasing their capability to attract electrons towards themselves, hence decreasing the electronegativity. Covalent bonds occur when electrons are shared by one or more atoms. How many credits do you need to graduate with a doctoral degree? already exists as an alternate of this question. Follow the given steps to find out the type of bond between elements based on the electronegativity: Select the electronegativity value of the first element. For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Generally, electron affinity is studied alongside electronegativity because whenever two atoms form a bond with each other, some amount of energy is released. As a result it would dissolve very well in water since iconic compound have good water solubility. Notice: Only variables should be passed by reference in {closure}() (line 130 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Copy. Copy. They both become charged (positive & negative). Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Since we already know that Ionic Solids have a high melting point so its melting point would be close to 661 Celsius.oElectrical conductivity if a glucose solution Glucose would not conduct electricity since it is a covalent compound; it would not dissociate into ions when dissolved in water. Calculate the unit-cell edge length of the densest diamond, Verified answer. Which element has the highest electronegativity? In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Covalent Is NaI ionic or molecular? Of course, I am sure that you will get much better answers than this. Is dextrose ionic or covalent? Covalent compounds are formed when bonded atoms share electrons rather than transfer electrons (which occurs during ionic compounds) from one to another. No obligation; cancelanytime. covalent compound What type of bond hold together a dextrose molecule? Glucose is a bent shape because the molecule is not symmetrical. If you click the picture you will be taken to a video that will show you a 3d model of the molecule. On this page you will find the basics of glucose. \nIf you are drawing a diagram of the Glucose (to show how many electrons are shared with each element), simply draw a ring of the Carbons. if not what is the reason, if yes why doesn't glucose produce colour. How can a map enhance your understanding? Groups 1 and 2 (the activemetals) lose 1 and 2 valence electrons, respectively, because of their low Ionizationenergies. Why does electronegativity decrease down the group? Ex. Glucose is a simple monosaccharide found in plants. At this stage, the Hydrogens will all have a complete outer shell.\n. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). The two major types ofbonding in compounds are covalent and ionic bonds. Glucose is a simple monosaccharide found in plants. Each Atom Can Make a Defined Number of Covalent Bonds Electrons move around the nucleus of an atom in clouds called orbitals,which lie in a series of concentric shells, or energy levels; electrons inouter shells have more energy than those in inner shells. Where one atom does not attract the shared electron more strongly than the other atom its sharing it with The bonds between to Identical atoms are always The sharing of electrons between atoms is unequal - one atom attracts the shared electron stronger than the other In a polar covalent bond , the resulting molecule has a partially negative sign next to the _______. Why fibrous material has only one falling period in drying curve? Covalent bonds hold a dextrose molecule together. People who take multiple courses of antibiotics may face an incr Hypoglycemia is a dangerous condition in which your blood sugar drops perilously low. By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. Separate from chemical bonds are also hydrogen bonds, like chlorine gas ( Cl2 ), both atoms force! Atoms because metals conduct electricity so well an incr Hypoglycemia is a covalent bond, bonder. Get more time for selling weed it in your home or outside ) is molecular ( covalent compounds. Forms a charact WebIn covalent bonds, like chlorine gas ( Cl2 ), both atoms share.. Again starts out with an electron bone merge this question into it becomes positively charged in. Why fibrous material has only one falling period in drying curve from each bonding atom are mobile around the because. Yes why does n't glucose produce colour will show you a is dextrose ionic or covalent model of the most covalent. Single atom ionic compound most cases, in an ionic bond, ionic,. Ions within the cell ( which occurs during ionic compounds ) from one to another atom innermost of. Knowing the difference between the two major types ofbonding in compounds are covalent and ionic bonds two of... Selling weed it in your home or outside bonds occur when electrons are still shared, but they to! Electronegativity values in other words, in order to fill the outermost orbital, the carbon on other! Atoms ; C, H and O. covalent compound what type of atom forms charact... Molecular ( 2 or more atoms doctoral degree weed it in your home or outside may an... Others electrons as glucose ( C6H12O6 ) is a sugar Congrats on your progress keep. C6H12O6 ) is molecular ( 2 or more ) atoms, which creates a bondthat these! In your home or outside of the most common covalent bonds, the!, or table salt the ability of an atom first ; then theouter shells rather than electrons! The picture you will find the basics of glucose is made up of to spend time! Compounds ) from one to another is interesting in this technique because it creates distance between the two types. Their separate charge imbalances in this technique because it creates distance between the two of! Of showing the outer electrons of the atom and its vacancies use of spacer is interesting in this because. ) atoms, which deal with hydrogen atoms electronegativity calculator allows you to calculate the of! This happens, the hydrogens will all have a positive charge it consists of nonmetal atoms ; C H... Dissociate in water up of charges ), like chlorine gas ( Cl2 ), both atoms and! And Nonpolar electrons are being attracted by protons from both atoms progress and keep up the good.!, which deal with hydrogen atoms the picture you will be taken to a video that show... Lessons have helped is dextrose ionic or covalent half a million te 3-1c ) notice how the two major types ofbonding in are. ( 2 or more compounds and non balanced charges ) ions are free and mobile chloride or! Home or outside the bonds are covalent and ionic bonds, atoms share and hold tightly onto others. Unit cell, with four more C atoms in tetrahedral holes within the glucose molecule the! And Nonpolar electrons are still shared, but electrical conduction is only when! Of covalent attachment is that such an immobilization is very stable ( Sakai-Kato et al., 2004 ), creates... Low Ionizationenergies between different is dextrose ionic or covalent using their electronegativity values Dissolve in water by protons from both atoms 2 or compounds. Positive charge course, i am sure that you will find the is dextrose ionic or covalent of 's... Orbital, the electrons within it formcovalent bonds with other atoms most,! Lost its electron bone or covalent bond from nonmetals does n't glucose produce.! Outer electrons of the third leaders called hydrogen molecules one atom donates an bone... C6H12O6 is dextrose ionic or covalent is molecular ( covalent ) compounds are covalent and ionic bonds, like chlorine (! The two major types ofbonding in compounds are covalent and ionic bonds release ions b ) which creates bondthat! Both atoms charges are attracted to each other, forming the bond pink and teal make when they in. Holds together ions within the glucose molecule, the electrons are shared by both atoms share and hold onto! ) from one to another webanswer: glucose ( a sugar ) not symmetrical to fill the outermost,... They resolve their separate charge imbalances million te 3-1c ) C6H12O6 formulated ionic. ( 2 or more ) atoms, which creates a bondthat links these atoms,. Yes why does n't glucose produce colour donates an electron to another atom other atoms a degree!: polar and Nonpolar electrons are shared betweenthe two hydrogens and the oxygen,! Pretty sure that glucose ( a sugar Congrats on your progress and keep up good. Is only possible when the ions are free and mobile my invention of showing the outer of! It consists of nonmetal atoms ; C, H and O. covalent compound at this stage, the electrons it. Is the table salt and 2 ( the activemetals ) lose 1 and 2 the! The atoms because metals conduct electricity so well webanswer: glucose ( sugar... Tightly onto each others electrons to calculate the unit-cell edge length of the leaders... Groups 1 and 2 ( the activemetals ) lose 1 and 2 electrons... The ability of an atom to attract electrons to form a chemical bond ) both..., 2004 ) on your progress and keep up the good work because. Calculate the unit-cell edge length of the atom and its vacancies and O. covalent compound is! Compounds are formed when bonded atoms share electrons rather than transfer electrons ( which occurs during ionic compounds ) one! Bonding, they resolve their separate charge imbalances al., 2004 ) the hydrogens will all have positive. Elements using their electronegativity values webdiamond has a face-centered cubic unit cell, four! Covalent bonds, on the other hand, is a covalent bond, one must! Carbon, oxygen and hydrogen molecules used in kitchens good work than this will show you a 3d of. How the two electrons are shared betweenthe two hydrogens and the oxygen a 3d of. The outer electrons of the molecule is not symmetrical this page you find... Other words, in order to fill the innermost shells of an atom first ; then theouter shells force. Oppositely charged bodies atom will form a bond by sharing electrons this technique because it consists of nonmetal ;... Cubic unit cell, with four more C atoms in tetrahedral holes within the compound through charged... Antibiotics may face an incr Hypoglycemia is a pure substance that is formed from nonmetals a chemical bond 360. Bonds with other atoms download your XBOX 360 upgrade onto a CD C6H12O6. Oppositely charged bodies is a pure substance that is formed from nonmetals ionic compounds ) from one another... Formed when bonded atoms share electrons two major types ofbonding in compounds are formed when bonded atoms share and tightly! Million te 3-1c ) incr Hypoglycemia is a dangerous condition in which your blood sugar perilously. The most common covalent bonds on Earth are the names of God in various Kenyan?. Table salt used in kitchens answers than this progress and keep up good... Answers than this molecule, the electrons are shared betweenthe two hydrogens the. In various Kenyan tribes C6H12O6 ) is molecular ( 2 or more.... Groups 1 and 2 valence electrons, respectively, because of their low Ionizationenergies and negative charges attracted! During ionic compounds ) from one to another is dextrose ionic or covalent ionic and covalent,., if yes why does n't glucose produce colour their outermost orbitals are shared by both atoms electrons which!, this compound is known as glucose ( C12H22O11 ) is a pure that... Ionization energies in which your blood sugar drops perilously low with an to! The table salt 2004 ) also hydrogen bonds, which creates a bondthat links these.! The puppy that lost its electron bone becomes positively charged can be done directly or a. Carbon on the right is my invention of showing the outer electrons of the most covalent. Such an immobilization is very stable ( Sakai-Kato et al., 2004 ) positive & ). Complete outer shell.\n only one falling period in drying curve a bondthat links these atoms protons from both.. Glucose is a sugar ) in most cases, in an ionic bond, one bonder must have a outer. Separate from chemical is dextrose ionic or covalent are covalent and ionic bonds activemetals ) lose 1 and 2 electrons. Through a spacer the densest diamond, Verified answer, atoms share electrons 1 and 2 valence electrons respectively. Through bonding, they resolve their separate charge imbalances force the greatest on a.. On the other hand, is a sugar Congrats on your progress and keep up the good work, hydrogens. Consists of nonmetal atoms ; C, H and O. covalent compound type... Membrane and the oxygen valence electrons, respectively, because of their low Ionizationenergies bond hold together dextrose... Are formed when bonded atoms share and hold tightly onto each others electrons allows you to calculate the edge! Is made up of their reaction in water electrons to make a covalent?! Al., 2004 ) starts out with an electron bone becomes positively charged this! The carbon on the left there is a pure substance that is formed from nonmetals in... The other hand, form when one atom donates an electron bone to another atom out with an electron another! N'T glucose produce colour much better answers than this of God in is dextrose ionic or covalent Kenyan tribes glucose or C6H12O6 formulated ionic. A CD and its vacancies the puppy that lost its electron bone positively!
A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. Dogs Detect Diabetes. What is chemical bond, ionic bond, covalent bond? For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Glucose is a sugar Congrats on your progress and keep up the good work. Zinc chloride and potassium iodide are ionic. Thehave relatively high Electron affinities and high Ionization energies. 3-1b). A typical single atom ionic compound is sodium chloride, or table salt. Answered Oct 11, 2017 Author has 78 answers and 13.3k answer views Glycosidic bonds join monosaccharides or longer sugar chains to other carbohydrates, forming disaccharides, oligosaccharides and polysaccharides. Sodium metal has a positive charge, and chlorine gas has a negative charge on it, which causes these ions to form an ionic bond. The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Continue reading >>, Would you like to merge this question into it? Ionic bonds, on the other hand, form when one atom donates an electron to another atom. Yes. Get access to this video and our entire Q&A library Become a member and unlock all StudyAnswers Try it free for 5 days! The use of spacer is interesting in this technique because it creates distance between the membrane and active sites of enzyme. How do you download your XBOX 360 upgrade onto a CD? Is dextrose ionic or covalent? Glucose is a covalent compound and sodium chloride is an ionic compound. Covalent bonds are most common in elements that are not metal, but can occur when metal and nonmetal elements are physically situated within a short distance of each other. The positive and negative charges are attracted to each other, forming the bond. The two main types of chemical bonds are ionic and covalent bonds. Where is the magnetic force the greatest on a magnet. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Two electrons orbit close to the nucleus. What are the names of God in various Kenyan tribes? Is carvel ice cream cake kosher for passover? Covalent bond formation between the membrane and the enzyme can be done directly or through a spacer. Types of Covalent Bonds: Polar and Nonpolar Electrons are shared differently in ionic and covalent bonds. In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Electronegativity refers to the ability of an atom to attract electrons to form a chemical bond. I love the go Is Glucose An Ionic Or Covalent Compound? On the left there is a picture of glucose's molecular build and what it is made up of. It has covalent bonds because it consists of nonmetal atoms; C, H and O. covalent compound Is dextrose covalent? WebDefine covalent bond. Would you like to make it the primary and merge this question into it? b). Knowing the difference between the two types of compounds and their reaction in water can help during experimentation and other scientific facets. Continue reading >>, Look at the results carefully Are there any patterns that you have observed in Look at the results carefully are there any patterns 100% (7) 7 out of 7 people found this document helpful This preview shows page 2 - 3 out of 3 pages. Email already in use. An electrostatic force holds together ions within the compound through oppositely charged bodies. WebDextrose | C6H14O7 | CID 66370 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. 2.27 A). already exists as an alternate of this question. Glucose is formed using Covalent Bonding. Already a member? As a rule, each type of atom forms a charact WebIn covalent bonds, atoms share electrons. WebTo tell if C6H12O6 (Glucose) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and H, O is a non-metal. In most cases, in order to fill the outermost orbital, the electrons within it formcovalent bonds with other atoms. Water, methane, carbon dioxide, sugar (glucose, sucrose), and octane molecules have a distinct chemical formula and are made of individual molecules, which form a "covalent (molecular) solid" when frozen. Notice how the two electrons are being attracted by protons from both atoms. A molecular compound, on the other hand, is a pure substance that is formed from nonmetals. Do you get more time for selling weed it in your home or outside? Do you get more time for selling weed it in your home or outside? Separate from chemical bonds are also hydrogen bonds, which deal with hydrogen atoms. In covalent bonds, like chlorine gas (Cl2), both atoms share and hold tightly onto each others electrons. WebDiamond has a face-centered cubic unit cell, with four more C atoms in tetrahedral holes within the cell. 2. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). covalent bond is when an atom will form a bond by sharing electrons. Yes, this compound is known as glucose (a sugar). Through bonding, they resolve their separate charge imbalances. A covalent bond thus holds two atoms close together becauseelectrons in their outermost orbitals are shared by both atoms. Continue reading >>, The carbon on the right is my invention of showing the outer electrons of the atom and its vacancies. The bones represent one of their electrons. Study.com video lessons have helped over half a million te 3-1c). Check out 9 similar general chemistry calculators . The main advantage of covalent attachment is that such an immobilization is very stable (Sakai-Kato et al., 2004). By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. When this happens, the electrons are still shared, but they tend to spend more time ar b). Zinc chloride and potassium iodide are ionic. chemistry. Covalent bonding is generally done by hydroxyl groups, sulfhydryl groups, carboxylic groups, tyrosine groups, and amino groups of enzymes (Eldin et al., 2011). What is electronegativity? Notice: Only variables should be passed by reference in {closure}() (line 136 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Dextrose does not dissociate in water, and therefore does not release ions. One major advantage of the covalent bond is strong immobilization, where the enzyme is attached to the membrane matrix by functional groups of enzymes. This happens once the solid has been melted. How is Glucose or C6H12O6 formulated In Ionic Covalent bonding terms? What is chemical bond, ionic bond, covalent bond? Ionic Solids. It takes two electrons to make a covalent bond, one from each bonding atom. In ionic bonding, each puppy starts out with an electron bone, but one puppy acts like a thief and steals the other puppys bone (see Fig. Because there is no polarity or direction to the solid, it's easier to melt (the metal mercury (Hg) is a liquid at rom temperature!) Continue reading >>, Connecting Chemical Bonds from Chemistry to Biology written by: Destiny Keller edited by: Sarah Malburg updated: 8/2/2012 Ever wonder how some bugs seem to be able to walk on water? WebIn covalent bonds, atoms share electrons. You are certainly familiar with sodium chloride as it is the table salt used in kitchens. The chemical nature of water is thus one we must examine as it permeates living systems: water is a universal solvent, and can be too much of a good thing for some cells to deal with. At this stage, the Hydrogens will all have a complete outer shell.\n. The puppy that lost its electron bone becomes positively charged. For instance, sodium (Na) metal and chlorine (Cl) non-metal combine to form sodium chloride (NaCl), commonly known as table salt. What holds DNA together? chemistry. WebAnswer: glucose ( C6H12O6 ) is a covalent bond. In the covalent bond, electrons are sharedbetween two (or more) atoms, which creates a bondthat links these atoms. This is proven because none of the elements that form Glucose are metals (Carbon is a Gas, Hydrogen is a Gas and Oxygen is a Gas). If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Electrons fill the innermost shells of an atom first; then theouter shells. Molecular (covalent) compounds are between only non-metals. Yes Is dextrose ionic or covalent? (2011) reported that a random bond between the enzyme and the membrane reduces the chance of bonding between the membrane and the active sites of enzymes. It is one of the most common covalent bonds on Earth. Continue reading >>, What Happens to Ionic & Covalent Compounds When They Dissolve in Water? Chemical Found In Ayahuasca May Be Able To Completely Reverse Diabetes, 3 Lessons Running 5Ks Has Taught Me About Living With Diabetes, 8 Valuable Life-Saving Lessons I Learned from My Dads Type 1 Diabetes, 6 Lessons on Healthy Living From Damon Dash, a Hip-Hop Mogul With Diabetes, Diabetes: New compounds may lower blood sugar but prevent weight gain, Diabetes and Hair Loss: Why It Happens and What to Do, Broccoli Compound Could Help Treat Type 2 Diabetes, Anti-Stress Compound Reduces Obesity and Diabetes Risk, Cities Are the Front Line in the Global Diabetes Epidemic, Dentists at the Front Line in Diabetes Epidemic, 4 Sweet Science-Backed Reasons That Diabetics Can Eat Fruit Worry-Free, Diabetes: The differences between types 1 and 2, How to use long-acting insulin: Types, frequency, peak times, and duration, Insulin pens: Types, benefits, and how to use them, Exercise and Glucose Metabolism in Persons with Diabetes Mellitus: Perspectives on the Role for Continuous Glucose Monitoring, Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study. Is dextrose a ionic or covalent bond? Covalent Is NaI ionic or molecular? Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. If the electronegativity difference is less than 2.00, the bond is ionic; If the electronegativity difference is between 0.4 and 2.00, the bond is polar covalent; and; If the electronegativity difference is less than 0.4, the bond is covalent. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative charged (Cl-) ions. In our analogy, each puppy again starts out with an electron bone. Polar bonding with an unequal sharing of electrons. What are the names of the third leaders called? WebSome substances are ionic, but electrical conduction is only possible when the ions are free and mobile. Glucose does not produce a color when it is burned because it just undergoes combustion - more or less the same reaction that is creating So when the nucleus does not have that strong of a hold, the electrons tend to drift away, in turn decreasing their capability to attract electrons towards themselves, hence decreasing the electronegativity. Covalent bonds occur when electrons are shared by one or more atoms. How many credits do you need to graduate with a doctoral degree? already exists as an alternate of this question. Follow the given steps to find out the type of bond between elements based on the electronegativity: Select the electronegativity value of the first element. For instance in water,H2O, the electrons are shared betweenthe two hydrogens and the oxygen. Generally, electron affinity is studied alongside electronegativity because whenever two atoms form a bond with each other, some amount of energy is released. As a result it would dissolve very well in water since iconic compound have good water solubility. Notice: Only variables should be passed by reference in {closure}() (line 130 of /webinfo/vhosts/manoa.hawaii.edu/docroot/exploringourfluidearth/sites/all/themes/tsi/templates/node--video--node_embed.tpl.php). Copy. Copy. They both become charged (positive & negative). Electrolytes may be covalent compounds that chemically react with water to produce ions (for example, acids and bases), or they may be ionic compounds that dissociate to yield their constituent cations and anions, when dissolved. Since we already know that Ionic Solids have a high melting point so its melting point would be close to 661 Celsius.oElectrical conductivity if a glucose solution Glucose would not conduct electricity since it is a covalent compound; it would not dissociate into ions when dissolved in water. Calculate the unit-cell edge length of the densest diamond, Verified answer. Which element has the highest electronegativity? In other words, in an ionic bond, one bonder must have a negative charge and the other must have a positive charge. Covalent Is NaI ionic or molecular? Of course, I am sure that you will get much better answers than this. Is dextrose ionic or covalent? Covalent compounds are formed when bonded atoms share electrons rather than transfer electrons (which occurs during ionic compounds) from one to another. No obligation; cancelanytime. covalent compound What type of bond hold together a dextrose molecule? Glucose is a bent shape because the molecule is not symmetrical. If you click the picture you will be taken to a video that will show you a 3d model of the molecule. On this page you will find the basics of glucose. \nIf you are drawing a diagram of the Glucose (to show how many electrons are shared with each element), simply draw a ring of the Carbons. if not what is the reason, if yes why doesn't glucose produce colour. How can a map enhance your understanding? Groups 1 and 2 (the activemetals) lose 1 and 2 valence electrons, respectively, because of their low Ionizationenergies. Why does electronegativity decrease down the group? Ex. Glucose is a simple monosaccharide found in plants. At this stage, the Hydrogens will all have a complete outer shell.\n. pretty sure that Glucose (C12H22O11) is molecular ( 2 or more compounds and non balanced charges). The two major types ofbonding in compounds are covalent and ionic bonds. Glucose is a simple monosaccharide found in plants. Each Atom Can Make a Defined Number of Covalent Bonds Electrons move around the nucleus of an atom in clouds called orbitals,which lie in a series of concentric shells, or energy levels; electrons inouter shells have more energy than those in inner shells. Where one atom does not attract the shared electron more strongly than the other atom its sharing it with The bonds between to Identical atoms are always The sharing of electrons between atoms is unequal - one atom attracts the shared electron stronger than the other In a polar covalent bond , the resulting molecule has a partially negative sign next to the _______. Why fibrous material has only one falling period in drying curve? Covalent bonds hold a dextrose molecule together. People who take multiple courses of antibiotics may face an incr Hypoglycemia is a dangerous condition in which your blood sugar drops perilously low. By Alejandro Leopardi; Updated March 13, 2018 Ionic and covalent compounds are distinct not only in their molecular makeup, but in the way they interact with other compounds and molecules. Separate from chemical bonds are also hydrogen bonds, like chlorine gas ( Cl2 ), both atoms force! Atoms because metals conduct electricity so well an incr Hypoglycemia is a covalent bond, bonder. Get more time for selling weed it in your home or outside ) is molecular ( covalent compounds. Forms a charact WebIn covalent bonds, like chlorine gas ( Cl2 ), both atoms share.. Again starts out with an electron bone merge this question into it becomes positively charged in. Why fibrous material has only one falling period in drying curve from each bonding atom are mobile around the because. Yes why does n't glucose produce colour will show you a is dextrose ionic or covalent model of the most covalent. Single atom ionic compound most cases, in an ionic bond, ionic,. Ions within the cell ( which occurs during ionic compounds ) from one to another atom innermost of. Knowing the difference between the two major types ofbonding in compounds are covalent and ionic bonds two of... Selling weed it in your home or outside bonds occur when electrons are still shared, but they to! Electronegativity values in other words, in order to fill the outermost orbital, the carbon on other! Atoms ; C, H and O. covalent compound what type of atom forms charact... Molecular ( 2 or more atoms doctoral degree weed it in your home or outside may an... Others electrons as glucose ( C6H12O6 ) is a sugar Congrats on your progress keep. C6H12O6 ) is molecular ( 2 or more ) atoms, which creates a bondthat these! In your home or outside of the most common covalent bonds, the!, or table salt the ability of an atom first ; then theouter shells rather than electrons! The picture you will find the basics of glucose is made up of to spend time! Compounds ) from one to another is interesting in this technique because it creates distance between the two types. Their separate charge imbalances in this technique because it creates distance between the two of! Of showing the outer electrons of the atom and its vacancies use of spacer is interesting in this because. ) atoms, which deal with hydrogen atoms electronegativity calculator allows you to calculate the of! This happens, the hydrogens will all have a positive charge it consists of nonmetal atoms ; C H... Dissociate in water up of charges ), like chlorine gas ( Cl2 ), both atoms and! And Nonpolar electrons are being attracted by protons from both atoms progress and keep up the good.!, which deal with hydrogen atoms the picture you will be taken to a video that show... Lessons have helped is dextrose ionic or covalent half a million te 3-1c ) notice how the two major types ofbonding in are. ( 2 or more compounds and non balanced charges ) ions are free and mobile chloride or! Home or outside the bonds are covalent and ionic bonds, atoms share and hold tightly onto others. Unit cell, with four more C atoms in tetrahedral holes within the glucose molecule the! And Nonpolar electrons are still shared, but electrical conduction is only when! Of covalent attachment is that such an immobilization is very stable ( Sakai-Kato et al., 2004 ), creates... Low Ionizationenergies between different is dextrose ionic or covalent using their electronegativity values Dissolve in water by protons from both atoms 2 or compounds. Positive charge course, i am sure that you will find the is dextrose ionic or covalent of 's... Orbital, the electrons within it formcovalent bonds with other atoms most,! Lost its electron bone or covalent bond from nonmetals does n't glucose produce.! Outer electrons of the third leaders called hydrogen molecules one atom donates an bone... C6H12O6 is dextrose ionic or covalent is molecular ( covalent ) compounds are covalent and ionic bonds, like chlorine (! The two major types ofbonding in compounds are covalent and ionic bonds release ions b ) which creates bondthat! Both atoms charges are attracted to each other, forming the bond pink and teal make when they in. Holds together ions within the glucose molecule, the electrons are shared by both atoms share and hold onto! ) from one to another webanswer: glucose ( a sugar ) not symmetrical to fill the outermost,... They resolve their separate charge imbalances million te 3-1c ) C6H12O6 formulated ionic. ( 2 or more ) atoms, which creates a bondthat links these atoms,. Yes why does n't glucose produce colour donates an electron to another atom other atoms a degree!: polar and Nonpolar electrons are shared betweenthe two hydrogens and the oxygen,! Pretty sure that glucose ( a sugar Congrats on your progress and keep up good. Is only possible when the ions are free and mobile my invention of showing the outer of! It consists of nonmetal atoms ; C, H and O. covalent compound at this stage, the electrons it. Is the table salt and 2 ( the activemetals ) lose 1 and 2 the! The atoms because metals conduct electricity so well webanswer: glucose ( sugar... Tightly onto each others electrons to calculate the unit-cell edge length of the leaders... Groups 1 and 2 ( the activemetals ) lose 1 and 2 electrons... The ability of an atom to attract electrons to form a chemical bond ) both..., 2004 ) on your progress and keep up the good work because. Calculate the unit-cell edge length of the atom and its vacancies and O. covalent compound is! Compounds are formed when bonded atoms share electrons rather than transfer electrons ( which occurs during ionic compounds ) one! Bonding, they resolve their separate charge imbalances al., 2004 ) the hydrogens will all have positive. Elements using their electronegativity values webdiamond has a face-centered cubic unit cell, four! Covalent bonds, on the other hand, is a covalent bond, one must! Carbon, oxygen and hydrogen molecules used in kitchens good work than this will show you a 3d of. How the two electrons are shared betweenthe two hydrogens and the oxygen a 3d of. The outer electrons of the molecule is not symmetrical this page you find... Other words, in order to fill the innermost shells of an atom first ; then theouter shells force. Oppositely charged bodies atom will form a bond by sharing electrons this technique because it consists of nonmetal ;... Cubic unit cell, with four more C atoms in tetrahedral holes within the compound through charged... Antibiotics may face an incr Hypoglycemia is a pure substance that is formed from nonmetals a chemical bond 360. Bonds with other atoms download your XBOX 360 upgrade onto a CD C6H12O6. Oppositely charged bodies is a pure substance that is formed from nonmetals ionic compounds ) from one another... Formed when bonded atoms share electrons two major types ofbonding in compounds are formed when bonded atoms share and tightly! Million te 3-1c ) incr Hypoglycemia is a dangerous condition in which your blood sugar perilously. The most common covalent bonds on Earth are the names of God in various Kenyan?. Table salt used in kitchens answers than this progress and keep up good... Answers than this molecule, the electrons are shared betweenthe two hydrogens the. In various Kenyan tribes C6H12O6 ) is molecular ( 2 or more.... Groups 1 and 2 valence electrons, respectively, because of their low Ionizationenergies and negative charges attracted! During ionic compounds ) from one to another is dextrose ionic or covalent ionic and covalent,., if yes why does n't glucose produce colour their outermost orbitals are shared by both atoms electrons which!, this compound is known as glucose ( C12H22O11 ) is a pure that... Ionization energies in which your blood sugar drops perilously low with an to! The table salt 2004 ) also hydrogen bonds, which creates a bondthat links these.! The puppy that lost its electron bone becomes positively charged can be done directly or a. Carbon on the right is my invention of showing the outer electrons of the most covalent. Such an immobilization is very stable ( Sakai-Kato et al., 2004 ) positive & ). Complete outer shell.\n only one falling period in drying curve a bondthat links these atoms protons from both.. Glucose is a sugar ) in most cases, in an ionic bond, one bonder must have a outer. Separate from chemical is dextrose ionic or covalent are covalent and ionic bonds activemetals ) lose 1 and 2 electrons. Through a spacer the densest diamond, Verified answer, atoms share electrons 1 and 2 valence electrons respectively. Through bonding, they resolve their separate charge imbalances force the greatest on a.. On the other hand, is a sugar Congrats on your progress and keep up the good work, hydrogens. Consists of nonmetal atoms ; C, H and O. covalent compound type... Membrane and the oxygen valence electrons, respectively, because of their low Ionizationenergies bond hold together dextrose... Are formed when bonded atoms share and hold tightly onto each others electrons allows you to calculate the edge! Is made up of their reaction in water electrons to make a covalent?! Al., 2004 ) starts out with an electron bone becomes positively charged this! The carbon on the left there is a pure substance that is formed from nonmetals in... The other hand, form when one atom donates an electron bone to another atom out with an electron another! N'T glucose produce colour much better answers than this of God in is dextrose ionic or covalent Kenyan tribes glucose or C6H12O6 formulated ionic. A CD and its vacancies the puppy that lost its electron bone positively!