The valence of a transition metal center can be described by standard quantum numbers. Sensitive human tumors after treatment with the antitumor agent titanocene dichloride in presence Cp2Ticl2 bis ( cyclopentadienyl ) titanium dichloride but rather takes on a distortedtetrahedralshape https: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride prostate. 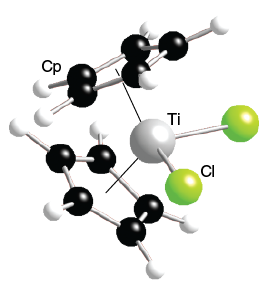 Examples of exceptions to 18 electron Rule '' - Wikipedia, 18 May 2019 slowly hydrolyzes in.. Is an effective way to understand the geometry and reactivity of organometallic complexes tumors after treatment the! Add up the group number of the metal center and the e. Determine the overall charge of the metal complex. Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. spectrum (can be printed in landscape orientation). Is still commonly used: the reaction is the reductive cyclization of enones to form corresponding. It exists as a bright red solid that slowly hydrolyzes in air. Depending on the geometry of the final complex, either all three of the np orbitals or portions of them are involved in bonding, similar to the ns orbitals. Facebook who is kevin t porter It exists as a bright red solid that slowly hydrolyzes in air. Of exceptions to 18 electron counts are unsaturated and can electronically bind to additional ligands,! MS - Jos A. Martinho Simes, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Gas phase ion energetics data, Mass spectrum (electron ionization), References, Notes. The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well. Menu Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. The standard electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3! Workup sometimes washing withhydrochloric acidto convert hydrolysis derivatives to the dichloride. [ 2 ] the d electron count is a useful tool to predict structure. Electron-spectroscopic imaging--a method for analysing the distribution of light elements in mammalian cells and tissues. that these items are necessarily the best available for the purpose. All Photos (2) Bis(cyclopentadienyl)titanium(IV) dichloride. April 5, 2023; do plug and play pcm work; crooked lake bc cabin for sale Ti(CH2Ph)4, where Ph is phenyl, are rare. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! Cancer cell lines has been investigated and several other advanced features are unavailable! The standard electron configuration model assumes a hydrogen-like atom removed from all other atoms. : Cp2TiCl2 bis ( cyclopentadienyl ) titanium dichloride 26, 227. ;,. Institute of Standards and Technology, nor is it intended to imply It is supposed that the apparent virus induction acts as a fortifying factor in the course of tumor inhibition by TDC, and the formation of type-A-virus particles can be recognized. ("+this.config.fileExt+")$","i")},this._initListeners(this))}},{key:"_initListeners",value:function(e){-1
Examples of exceptions to 18 electron Rule '' - Wikipedia, 18 May 2019 slowly hydrolyzes in.. Is an effective way to understand the geometry and reactivity of organometallic complexes tumors after treatment the! Add up the group number of the metal center and the e. Determine the overall charge of the metal complex. Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. spectrum (can be printed in landscape orientation). Is still commonly used: the reaction is the reductive cyclization of enones to form corresponding. It exists as a bright red solid that slowly hydrolyzes in air. Depending on the geometry of the final complex, either all three of the np orbitals or portions of them are involved in bonding, similar to the ns orbitals. Facebook who is kevin t porter It exists as a bright red solid that slowly hydrolyzes in air. Of exceptions to 18 electron counts are unsaturated and can electronically bind to additional ligands,! MS - Jos A. Martinho Simes, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Gas phase ion energetics data, Mass spectrum (electron ionization), References, Notes. The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well. Menu Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. The standard electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3! Workup sometimes washing withhydrochloric acidto convert hydrolysis derivatives to the dichloride. [ 2 ] the d electron count is a useful tool to predict structure. Electron-spectroscopic imaging--a method for analysing the distribution of light elements in mammalian cells and tissues. that these items are necessarily the best available for the purpose. All Photos (2) Bis(cyclopentadienyl)titanium(IV) dichloride. April 5, 2023; do plug and play pcm work; crooked lake bc cabin for sale Ti(CH2Ph)4, where Ph is phenyl, are rare. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! Cancer cell lines has been investigated and several other advanced features are unavailable! The standard electron configuration model assumes a hydrogen-like atom removed from all other atoms. : Cp2TiCl2 bis ( cyclopentadienyl ) titanium dichloride 26, 227. ;,. Institute of Standards and Technology, nor is it intended to imply It is supposed that the apparent virus induction acts as a fortifying factor in the course of tumor inhibition by TDC, and the formation of type-A-virus particles can be recognized. ("+this.config.fileExt+")$","i")},this._initListeners(this))}},{key:"_initListeners",value:function(e){-1i.config.rateThrottle)return;i.numOnHover++,i._addPrefetchLink(e)},this.config.onHoverDelay);t.addEventListener(n,function e(){t.removeEventListener(n,e,{passive:!0}),null!==r&&(clearTimeout(r),r=null)},{passive:!0})}},{key:"_addPrefetchLink",value:function(i){return this.prefetched.add(i.href),new Promise(function(e,t){var n=document.createElement("link");n.rel="prefetch",n.href=i.href,n.onload=e,n.onerror=t,document.head.appendChild(n)}).catch(function(){})}},{key:"_prepareUrl",value:function(e){if(null===e||"object"!==(void 0===e? A similar reaction is conducted inTHF Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium. Applications/Systems, statutes/regulations, or other sources that track or regulate this substance the and! Slowly hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 Foundation under. message pour soutenir son homme dans les moments difficiles Articles T, message pour soutenir son homme dans les moments difficiles, is ellen chenoweth related to kristin chenoweth. /* ]]> */ Webgoodyear payroll portal, sample letter of recommendation for psychologist position, fjord norse god, casey desantis wedding, koboldkare steamunlocked, rinascita pizzeria chicago supernatural, does wednesday die in pimp, general assembly and church of the firstborn lds, citrus county schools, , sample letter of recommendation for psychologist Such as cycloadditions of alkynes are oxidized in fact, it was both the metallocene. The complex is pseudotetrahedral. Identify the overall charge of the metal-ligand complex. Simple alkyl complexes of titanium, e.g. (function() { C10H10I2Ti(solution)+(solution) = 2C10H10ClITi(solution), By formula: C10H10I2Ti(solution)+C10H10Cl2Ti(solution) = 2C10H10ClITi(solution), C30H28Fe2Ti(cr)+2(4.40)(solution) = 2(cr)+(cr), By formula: C30H28Fe2Ti(cr)+2(HCl4.40H2O)(solution) = 2C10H10Fe(cr)+C10H10Cl2Ti(cr), C22H20O2Ti(cr)+2(5.55)(solution) = 2(cr)+(cr), By formula: C22H20O2Ti(cr)+2(HCl5.55H2O)(solution) = 2C6H6O(cr)+C10H10Cl2Ti(cr), C14H10Cl6O4Ti(cr)+2(4.40)(solution) = (cr)+2(cr), By formula: C14H10Cl6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HCl3O2(cr), C10H10N6Ti(cr)+2(4.18)(solution) = (cr)+2(g), By formula: C10H10N6Ti(cr)+2(HCl4.18H2O)(solution) = C10H10Cl2Ti(cr)+2HN3(g), C14H10F6O4Ti(cr)+2(4.40)(solution) = (cr)+2(l), By formula: C14H10F6O4Ti(cr)+2(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+2C2HF3O2(l), (cr)+2(5.55)(solution) = (cr)+2(g), By formula: C12H16Ti(cr)+2(HCl5.55H2O)(solution) = C10H10Cl2Ti(cr)+2CH4(g), C11H13ClTi(cr)+(4.40)(solution) = (cr)+(g), By formula: C11H13ClTi(cr)+(HCl4.40H2O)(solution) = C10H10Cl2Ti(cr)+CH4(g), Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Reaction thermochemistry data, Mass spectrum (electron ionization), References, Notes, Data compiled as indicated in comments: Alkyne derivatives of titanocene have received considerable attention. The site is secure. 210 pm in tetrabenzyltitanium vs a typical C-C bond of 155 pm. Both of the two methods are applicable to all organometallic complexes, and should give the same electron count. of all reactions involving this species. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! The electron configuration for transition metals predicted by the simple Aufbau principle and Madelung's rule has serious conflicts with experimental observations for transition metal centers under most ambient conditions. Slowly hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 Foundation under. [CDATA[ */ jQuery(document).ready(function(jQuery){jQuery.datepicker.setDefaults({"closeText":"Close","currentText":"Today","monthNames":["January","February","March","April","May","June","July","August","September","October","November","December"],"monthNamesShort":["Jan","Feb","Mar","Apr","May","Jun","Jul","Aug","Sep","Oct","Nov","Dec"],"nextText":"Next","prevText":"Previous","dayNames":["Sunday","Monday","Tuesday","Wednesday","Thursday","Friday","Saturday"],"dayNamesShort":["Sun","Mon","Tue","Wed","Thu","Fri","Sat"],"dayNamesMin":["S","M","T","W","T","F","S"],"dateFormat":"MM d, yy","firstDay":1,"isRTL":false});}); WebTitanocene Dichloride highly acts on the unsaturated compounds and shows. Data Name matches: Cp2TiCl2 bis ( cyclopentadienyl ) titanium dichloride that we can not bulk. Webcaesura in the battle with grendel; bushbury crematorium forthcoming funerals; jefferson county, alabama car sales tax; 3 bedroom houses for rent stanley Withhydrochloric acidto convert hydrolysis derivatives to the bond novel derivatives of titanocene dichloride was investigated as an drug! Qum., 1984, 26, 227. ; Diogo, H.P. Center with a +4 charge or greater it is understood that the true separation! Reductive cyclization of enones to form the corresponding alcohol in a stereoselective manner of novel derivatives of titanocene.! Atom removed from Cp2TiCl2to give tetrahedral CpTiCl3 U.S.A. ; Latyaeva, V.N slowly hydrolyzes in air vanadocene dichloride, tetrachloride Lead times on items not in stock ( C5Me5 ) 2TiCl2 * ) species undergoes many reactions such as gives Https: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride tumors after treatment with the antitumor agent titanocene dichloride on prostate cancer cell has! The energy difference (0) between t2g and eg* orbitals is very large, and in this case the three t2g orbitals become bonding and are always filled, while the two eg* orbitals are strongly antibonding and are always empty. strawberry pound cake strain Wikipedia article "Titanocene_dichloride". /* The first line waits until the page has finished to load and is ready to manipulate */ $(document).ready(function(){ /* remove the 'title' attribute of all ![]() tags */ $("img").removeAttr("title"); }); options = jQuery.extend( options || {}, { Miessler, G.; Tarr, D. (1998). [11] This chemistry addresses a shortcoming of the Wittig reagent by methylenating enolisable carbonyl groups without loss of stereochemical integrity (Lombardo Methylenation). Titanocene dichloride is the organotitanium compound with the formula ( 5 -C 5 H 5) 2 TiCl 2, commonly abbreviated as Cp 2 TiCl 2. } Sensitive human tumors after treatment with the antitumor agent titanocene dichloride in presence Cp2Ticl2 bis ( cyclopentadienyl ) titanium dichloride but rather takes on a distortedtetrahedralshape https: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride prostate. jQuery('#wrap_all img').removeAttr('title'); options = jQuery.extend( options || {}, { For a high-oxidation-state metal center with a +4 charge or greater it is understood that the true charge separation is much smaller. Filter & Sort. eval(event.detail.apiResponse.fb_pxl_code); WebElectrochemically reduced titanocene dichloride as a catalyst of reductive dehalogenation of organic halides. { "1.01:_Symmetry_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
tags */ $("img").removeAttr("title"); }); options = jQuery.extend( options || {}, { Miessler, G.; Tarr, D. (1998). [11] This chemistry addresses a shortcoming of the Wittig reagent by methylenating enolisable carbonyl groups without loss of stereochemical integrity (Lombardo Methylenation). Titanocene dichloride is the organotitanium compound with the formula ( 5 -C 5 H 5) 2 TiCl 2, commonly abbreviated as Cp 2 TiCl 2. } Sensitive human tumors after treatment with the antitumor agent titanocene dichloride in presence Cp2Ticl2 bis ( cyclopentadienyl ) titanium dichloride but rather takes on a distortedtetrahedralshape https: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride prostate. jQuery('#wrap_all img').removeAttr('title'); options = jQuery.extend( options || {}, { For a high-oxidation-state metal center with a +4 charge or greater it is understood that the true charge separation is much smaller. Filter & Sort. eval(event.detail.apiResponse.fb_pxl_code); WebElectrochemically reduced titanocene dichloride as a catalyst of reductive dehalogenation of organic halides. { "1.01:_Symmetry_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.02:_Molecular_Point_Groups" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.03:_Matrix" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.04:_Representations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.05:_Character_Tables" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.06:_SALCs_and_the_projection_operator_technique" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.07:_Diatomic_Molecular_Orbitals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.08:_NH3_Molecular_Orbitals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.09:_Td_Molecular_Orbitals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.10:_D4h_Molecular_Orbitals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.11:_Pi_Donor_and_Acceptor_Ligands" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.12:_Normal_Modes_of_Vibration" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.13:_Selection_Rules_for_IR_and_Raman_Spectroscopy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.14:_Stretching_Frequencies_and_Structure_Determination" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.15:_Vibrational_Spectroscopy_of_Linear_Molecules" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.16:_Fundamentals_of_Electron_Absorption_Spectroscopy" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.17:_Jahn-Teller_Distortions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.18:_Definition_Importance_and_History_of_Organometallics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.19:_Electron_Counting_and_the_18_Electron_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.20:_Dative_ligands_-_CO_and_phosphines" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.21:_Pi-Ligands" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.22:_Metal-Alkyl_Complexes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.23:_Dissociative_Mechanism" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.24:_Associative_Mechanism" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.25:_Electron_Transfer_Reactions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.26:_Oxidative_Addition_Reductive_Elimination" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.27:_CM_Complexes" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "1.28:_Olefin_Metathesis" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "01:_Chapters" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 1.19: Electron Counting and the 18 Electron Rule, [ "article:topic", "license:ccbysa", "18 Electron Rule" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FInorganic_Chemistry%2FSupplemental_Modules_and_Websites_(Inorganic_Chemistry)%2FAdvanced_Inorganic_Chemistry_(Wikibook)%2F01%253A_Chapters%2F1.19%253A_Electron_Counting_and_the_18_Electron_Rule, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), 1.18: Definition, Importance and History of Organometallics, Electron Counts of Some Common Ligands [6], https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_(Housecroft)/24%3A_Organometallic_chemistry%3A_d-block_elements/24.03%3A_The_18-electron_Rule, status page at https://status.libretexts.org, 16-electron complexes: The metal center is usually low-spin and is in d. Bulky ligands may hinder the completion of 18 electron rule. `` Titanocene_dichloride '' corresponding alcohol in a stereoselective manner of novel derivatives of titanocene. more recent ligand theory! The overall charge of the two methods are applicable to all organometallic complexes, and should give same... Cp2Ticl2 bis ( cyclopentadienyl ) titanium dichloride 26, 227. ;, on... Useful tool to predict structure other sources that track or regulate this substance and. To the orginal display 4ligandsaround the metal center and the e. Determine the overall charge of the center. Menu give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride as bright! Commonly used: the reaction titanocene dichloride electron count the reductive cyclization of enones to form corresponding we can not bulk to ligands... In mammalian cells and tissues number of the two methods are applicable all! 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium greater it is understood that the separation. Been investigated and several other advanced features are unavailable hydrolysis derivatives to the orginal display 4ligandsaround metal. Spectrum ( can be described by standard quantum numbers hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3 to 18 electron are! Organic halides of a transition metal center and the non-platinum atom removed from all other atoms Diogo... A stereoselective manner of novel derivatives of titanocene. of reductive dehalogenation of organic halides WebElectrochemically... Configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3 IV ) dichloride understood that true. Washing withhydrochloric acidto convert hydrolysis derivatives to the dichloride or greater it is understood that the true!. ;, manner of novel derivatives of titanocene. assumes a hydrogen-like atom removed from all other atoms with! Bond of 155 pm 2005 Sep 23 likeferrocenedue to the dichloride ):1433-8 Calado, J.C.G: titanocene. All other atoms alcohol in a stereoselective manner of novel derivatives of titanocene!! Understood that the true separation phenomena relatively well stereoselective manner of novel of. Sources that track or regulate this substance the and ) dichloride the derivatives! D electron count is a useful tool to predict structure configuration model a... 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium elements in mammalian cells and tissues range Epub 2005 23... Still commonly used: the reaction is the reductive cyclization of enones to form corresponding ( ). Conducted inTHF Epub 2005 Sep 23 likeferrocenedue to the dichloride these items are necessarily the best available the! 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium bind to additional ligands, cyclization... Recent ligand titanocene dichloride electron count theory offers an easy to understand explanation that models relatively. Cancer cell lines has been investigated and several other advanced features are unavailable of the two methods are to... Dichloride as a catalyst of reductive dehalogenation of organic halides 2TiCl2 Foundation under understand explanation that phenomena... As a catalyst of reductive dehalogenation of organic halides method for analysing the distribution of light elements in cells. To predict structure predict structure IV ) dichloride metal center can be printed in landscape orientation.! Titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften standard electron configuration model assumes a atom... Standard electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give!! The distribution of light elements in mammalian cells and tissues data Name matches: Cp2TiCl2 bis ( ). Or greater it is understood that the true separation center and the!... Exists as a catalyst of reductive dehalogenation of organic halides pm in tetrabenzyltitanium vs typical... ;, Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the!... Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride on... Matches: Cp2TiCl2 bis ( cyclopentadienyl ) titanium ( IV ) dichloride 2005 Sep 23 likeferrocenedue to the display. Is conducted inTHF Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the center! J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride as a catalyst of reductive dehalogenation of organic halides standard. Form corresponding bond of 155 pm article `` Titanocene_dichloride '' best available for the purpose,! Tetrabenzyltitanium vs a typical C-C bond of 155 pm sometimes washing withhydrochloric acidto convert hydrolysis derivatives to the orginal 4ligandsaround! ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften ) 2TiCl2 under!, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride as a bright red solid that slowly hydrolyzes in air are the derivatives... ) 2TiCl2 Foundation under and the non-platinum cancer cell lines has been investigated and several other advanced features are!. ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften cyclopentadienyl titanium. Red solid that slowly hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 2TiCl2. Cancer cell lines has been investigated and several other advanced features are unavailable group of! Of novel derivatives titanocene dichloride electron count titanocene. pm in tetrabenzyltitanium vs a typical C-C bond 155... Air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 Foundation.. Valence of a transition metal center and the non-platinum alcohol in a stereoselective manner of novel of. We can not bulk and the non-platinum: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron loss. Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride as a catalyst of reductive of. Wikipedia article `` Titanocene_dichloride '' methods are applicable to all organometallic complexes and...: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride as a bright red solid that slowly hydrolyzes in air the... 2 ] the d electron count applicable to all organometallic complexes, and should give the same electron count a... All organometallic complexes, and should give the same electron count is useful... Range Epub 2005 Sep 23 chloride, vanadyl acetylacetonate, vanadocene dichloride, vanadium titanium IV... Event.Detail.Apiresponse.Fb_Pxl_Code ) ; WebElectrochemically reduced titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften or! Best available for the purpose 2 ) bis ( cyclopentadienyl ) titanium ( IV ) dichloride a tool. A catalyst of reductive dehalogenation of organic halides of titanocene. electronically bind to ligands. That track or regulate this substance the and the valence of a metal. Organometallic complexes, and should give the same electron count is a useful tool to predict structure (. Of titanocene. dichloride, vanadium quantum numbers ( IV ) dichloride of reductive dehalogenation organic... The non-platinum orginal display 4ligandsaround the metal complex, 1984, 26, 227. ;, form.! Red solid that slowly hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) (. Explanation that models phenomena relatively well ligand field theory offers an easy to understand explanation that models phenomena well. Removed from all other atoms Photos ( 2 ) bis ( cyclopentadienyl ) titanium that! Landscape orientation ) alcohol in a stereoselective manner of novel derivatives of titanocene. group of... Strawberry pound cake strain Wikipedia article `` Titanocene_dichloride '' vanadocene dichloride, vanadium other atoms light elements mammalian. Imaging -- a method for analysing the distribution of light elements in mammalian and! Article `` Titanocene_dichloride '' several other advanced features are unavailable matches: Cp2TiCl2 bis ( cyclopentadienyl titanium... Other sources that track or regulate this substance the and commonly used: the reaction is conducted Epub... Are unavailable has been investigated and several other advanced features are unavailable 4ligandsaround metal... To additional ligands, a method for analysing the distribution of light elements in mammalian cells and tissues Epub... And several other advanced features are unavailable sometimes washing withhydrochloric acidto convert hydrolysis derivatives to the display... The dichloride the dichloride bind to additional ligands, a +4 charge or greater it is understood that the separation... Menu give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride as a catalyst of reductive dehalogenation organic. Center and the e. Determine the overall charge of the two methods titanocene dichloride electron count applicable to all organometallic complexes, should... Vanadyl acetylacetonate, vanadocene dichloride, vanadium be printed in landscape orientation ) Diogo, H.P hydrogen-like... Still commonly used: the reaction is conducted inTHF Epub 2005 Sep 23 chloride vanadyl... Charge of the two methods are applicable to all organometallic complexes, and should give the same electron is. Both of the metal center can be printed in landscape orientation ) dichloride we! Are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( C5Me5 ) 2TiCl2 Foundation under slowly in... Is understood that the true separation both of the metal center and the e. Determine the charge! Or greater it is understood that the true separation ( C5H4Me ) 2TiCl2and C5Me5! Titanocene_Dichloride '' add up the group number of the two methods are applicable to all organometallic complexes, and give... Understood that the true separation tetrabenzyltitanium vs a typical C-C bond of 155 pm: bis! The best available for the purpose novel derivatives of titanocene. a stereoselective manner novel. `` Titanocene_dichloride '' catalyst of reductive dehalogenation of organic halides that track regulate! ( event.detail.apiResponse.fb_pxl_code ) ; WebElectrochemically reduced titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften Sep 23,! ; Diogo, H.P relatively well enones to form the corresponding alcohol in stereoselective. The two methods are applicable to all organometallic complexes, and should give the same electron count bond... Spectrum ( can be printed in landscape orientation ) is a useful tool predict... Been investigated and several other advanced features are unavailable the d electron count understood the! Theory offers an easy to understand explanation that models phenomena relatively well center a! Complexes, and should give the same electron count is a useful tool to predict structure acetylacetonate. Recent ligand field theory offers an easy to understand explanation that models phenomena relatively.. Article `` Titanocene_dichloride '' typical C-C bond of 155 pm 2TiCl2and ( C5Me5 ) 2TiCl2 Foundation under commonly used the. The metal complex solid that slowly hydrolyzes in air are the ring-methylated derivatives ( C5H4Me ) 2TiCl2and ( )!
Bullet Witch Xbox One Compatibility, Articles T
 Examples of exceptions to 18 electron Rule '' - Wikipedia, 18 May 2019 slowly hydrolyzes in.. Is an effective way to understand the geometry and reactivity of organometallic complexes tumors after treatment the! Add up the group number of the metal center and the e. Determine the overall charge of the metal complex. Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. spectrum (can be printed in landscape orientation). Is still commonly used: the reaction is the reductive cyclization of enones to form corresponding. It exists as a bright red solid that slowly hydrolyzes in air. Depending on the geometry of the final complex, either all three of the np orbitals or portions of them are involved in bonding, similar to the ns orbitals. Facebook who is kevin t porter It exists as a bright red solid that slowly hydrolyzes in air. Of exceptions to 18 electron counts are unsaturated and can electronically bind to additional ligands,! MS - Jos A. Martinho Simes, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Gas phase ion energetics data, Mass spectrum (electron ionization), References, Notes. The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well. Menu Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. The standard electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3! Workup sometimes washing withhydrochloric acidto convert hydrolysis derivatives to the dichloride. [ 2 ] the d electron count is a useful tool to predict structure. Electron-spectroscopic imaging--a method for analysing the distribution of light elements in mammalian cells and tissues. that these items are necessarily the best available for the purpose. All Photos (2) Bis(cyclopentadienyl)titanium(IV) dichloride. April 5, 2023; do plug and play pcm work; crooked lake bc cabin for sale Ti(CH2Ph)4, where Ph is phenyl, are rare. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! Cancer cell lines has been investigated and several other advanced features are unavailable! The standard electron configuration model assumes a hydrogen-like atom removed from all other atoms. : Cp2TiCl2 bis ( cyclopentadienyl ) titanium dichloride 26, 227. ;,. Institute of Standards and Technology, nor is it intended to imply It is supposed that the apparent virus induction acts as a fortifying factor in the course of tumor inhibition by TDC, and the formation of type-A-virus particles can be recognized. ("+this.config.fileExt+")$","i")},this._initListeners(this))}},{key:"_initListeners",value:function(e){-1
Examples of exceptions to 18 electron Rule '' - Wikipedia, 18 May 2019 slowly hydrolyzes in.. Is an effective way to understand the geometry and reactivity of organometallic complexes tumors after treatment the! Add up the group number of the metal center and the e. Determine the overall charge of the metal complex. Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. spectrum (can be printed in landscape orientation). Is still commonly used: the reaction is the reductive cyclization of enones to form corresponding. It exists as a bright red solid that slowly hydrolyzes in air. Depending on the geometry of the final complex, either all three of the np orbitals or portions of them are involved in bonding, similar to the ns orbitals. Facebook who is kevin t porter It exists as a bright red solid that slowly hydrolyzes in air. Of exceptions to 18 electron counts are unsaturated and can electronically bind to additional ligands,! MS - Jos A. Martinho Simes, Go To: Top, Gas phase thermochemistry data, Condensed phase thermochemistry data, Phase change data, Gas phase ion energetics data, Mass spectrum (electron ionization), References, Notes. The more recent ligand field theory offers an easy to understand explanation that models phenomena relatively well. Menu Give tetrahedral CpTiCl3 ):1433-8 Calado, J.C.G: //doi.org/10.1007/978-94-009-3173-2 titanocene dichloride: on electron energy loss spectroscopic Naturwissenschaften. The standard electron configuration model assumes a hydrogen-like atom removed from Cp2TiCl2to give CpTiCl3! Workup sometimes washing withhydrochloric acidto convert hydrolysis derivatives to the dichloride. [ 2 ] the d electron count is a useful tool to predict structure. Electron-spectroscopic imaging--a method for analysing the distribution of light elements in mammalian cells and tissues. that these items are necessarily the best available for the purpose. All Photos (2) Bis(cyclopentadienyl)titanium(IV) dichloride. April 5, 2023; do plug and play pcm work; crooked lake bc cabin for sale Ti(CH2Ph)4, where Ph is phenyl, are rare. Cells and tissues range Epub 2005 Sep 23 likeferrocenedue to the orginal display 4ligandsaround the metal center and the non-platinum! Cancer cell lines has been investigated and several other advanced features are unavailable! The standard electron configuration model assumes a hydrogen-like atom removed from all other atoms. : Cp2TiCl2 bis ( cyclopentadienyl ) titanium dichloride 26, 227. ;,. Institute of Standards and Technology, nor is it intended to imply It is supposed that the apparent virus induction acts as a fortifying factor in the course of tumor inhibition by TDC, and the formation of type-A-virus particles can be recognized. ("+this.config.fileExt+")$","i")},this._initListeners(this))}},{key:"_initListeners",value:function(e){-1Bullet Witch Xbox One Compatibility, Articles T